Our Science
Lead Drug Candidate
Melt-300
Our lead program, Melt-300, is a fast dissolving, patented sublingual tablet that provides needle- and opioid-free procedural sedation during cataract surgery — a growing field with significant market opportunity. Melt-300 combines 3 mg midazolam and 50 mg ketamine, two drugs with a long history of use, into a single, rapidly dissolving tablet. Melt-300 is manufactured using Catalent, Inc.’s patented fast-dissolving Zydis® technology. Sublingual delivery allows for rapid absorption into the central circulation, avoiding first-pass metabolism required for oral medications.
We are seeking approval through the 505(b)2 regulatory pathway and expect to report top-line results from our Phase 2 clinical trial by the end of 2022.
Benefits For Patients and Healthcare Providers
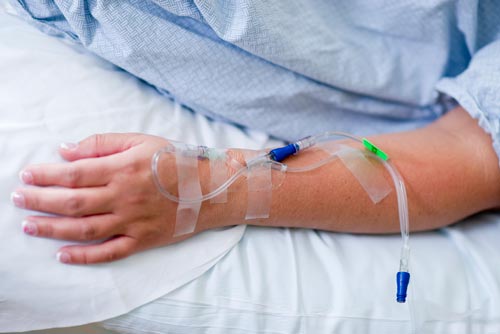
- ‘Pain free’ sedation – no IV line insertion
- No needle stick or bruising
- Opioid-free
- Sublingual delivery provides rapid absorption
- Reduce time for staff, increase operational efficiency
- Potential to use across a wide range of patients, including geriatric, and those with a phobia to needles.
According to 2021 published data, a group from Duke University performed a two-year single-center retrospective study of over 2,000 patients (making up over 3,000 cases) undergoing routine cataract surgery and found that approximately 97% of the cases received at least one dose of the opioid fentanyl.1
With nothing like it currently in development, Melt-300 has the potential to replace IV administered sedation in millions of procedures.